
We inform you that the privacy policy at the remote site can be different from the covid19vaccinejanssen.com website. Download a blank covid vaccine card in pdf form. Guía técnica 10 de agosto 2021. Since the first positive results on vaccines have come out, a lot of people have asked me if i think everyone should take them? It may come sooner than we think. How to fill it out so it looks legitimate. The janssen covid‑19 vaccine has not been approved or licensed by the u.s. Acip lifts pause on the administration of janssen/johnson & johnson vaccines. Food and drug administration issued an emergency use authorization (eua) for the third vaccine for the prevention. We inform you that the privacy policy at the remote site can be different from the covid19vaccinejanssen.com website. The cause is not necessarily related to the vaccine.
If either of these conditions exists, do not administer the vaccine. The janssen covid‑19 vaccine has not been approved or licensed by the u.s. The emergency use of this product is only.

It was authorized in the eu on march 11 but the rollout of the shots has not started yet.
Authorized the janssen shot in late february and has administered nearly 5 million doses as of. Unless approved or licensed by the relevant health authority, the product is investigational and its safety and efficacy have not been established. This cma has similar requirements to that granted by the mhra. Acip lifts pause on the administration of janssen/johnson & johnson vaccines. Since the first positive results on vaccines have come out, a lot of people have asked me if i think everyone should take them? Guía técnica 10 de agosto 2021. It was authorized in the eu on march 11 but the rollout of the shots has not started yet. If either of these conditions exists, do not administer the vaccine. It may come sooner than we think. Here is how to make a counterfeit vaccination record for you and your family. An adverse event is a medical incident that may occur following immunization. Food and drug administration issued an emergency use authorization (eua) for the third vaccine for the prevention. The emergency use of this product is only. On february 27, 2021, the u.s. Food and drug administration (fda), but has been authorized by fda through an emergency use authorization (eua) for active immunization to prevent coronavirus disease 2019 (covid‑19) in individuals 18 years of.
Food and drug administration (fda), but has been authorized by fda through an emergency use authorization (eua) for active immunization to prevent coronavirus disease 2019 (covid‑19) in individuals 18 years of. It was authorized in the eu on march 11 but the rollout of the shots has not started yet. An adverse event is a medical incident that may occur following immunization. If either of these conditions exists, do not administer the vaccine. The janssen covid‑19 vaccine has not been approved or licensed by the u.s.
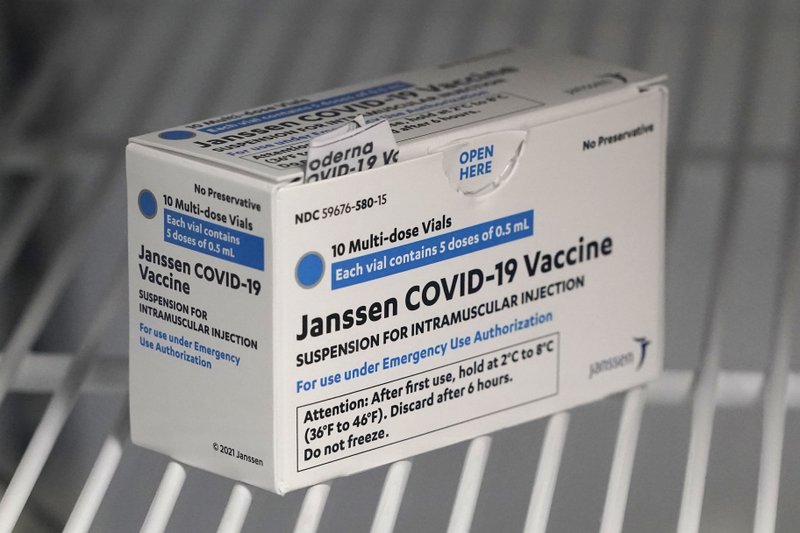
The cause is not necessarily related to the vaccine.
Food and drug administration issued an emergency use authorization (eua) for the third vaccine for the prevention. Here is how to make a counterfeit vaccination record for you and your family. The cause is not necessarily related to the vaccine. The emergency use of this product is only. If either of these conditions exists, do not administer the vaccine. Guía técnica 10 de agosto 2021. The janssen covid‑19 vaccine has not been approved or licensed by the u.s. Authorized the janssen shot in late february and has administered nearly 5 million doses as of. How to fill it out so it looks legitimate. An adverse event is a medical incident that may occur following immunization. It was authorized in the eu on march 11 but the rollout of the shots has not started yet. Acip lifts pause on the administration of janssen/johnson & johnson vaccines. Food and drug administration (fda), but has been authorized by fda through an emergency use authorization (eua) for active immunization to prevent coronavirus disease 2019 (covid‑19) in individuals 18 years of.
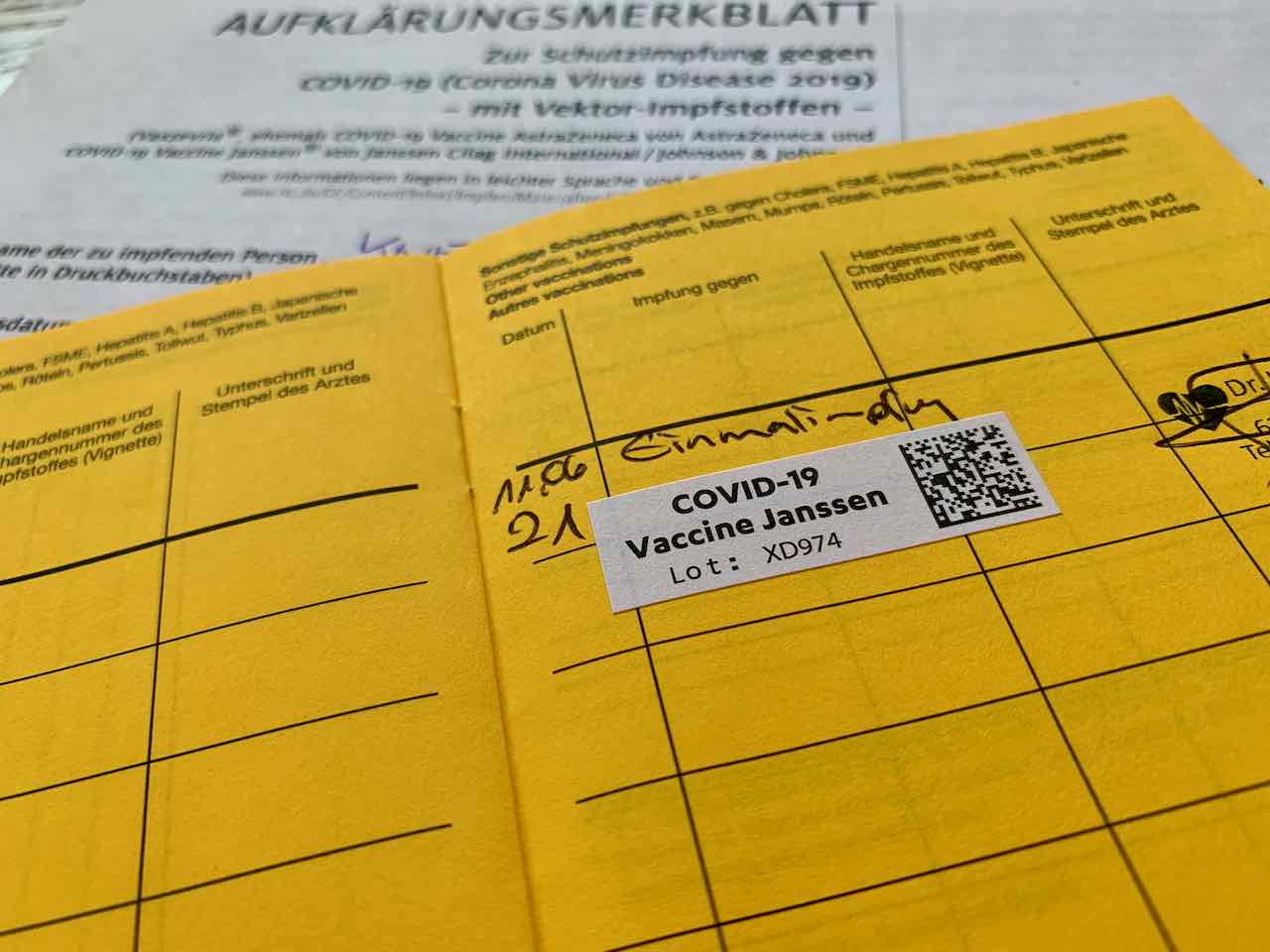
How to fill it out so it looks legitimate. The janssen covid‑19 vaccine has not been approved or licensed by the u.s. Here is how to make a counterfeit vaccination record for you and your family.

Here is how to make a counterfeit vaccination record for you and your family.
Arrangements for the supply of the vaccine are the responsibility of national authorities. Food and drug administration issued an emergency use authorization (eua) for the third vaccine for the prevention. Unless approved or licensed by the relevant health authority, the product is investigational and its safety and efficacy have not been established. The janssen covid‑19 vaccine has not been approved or licensed by the u.s. If either of these conditions exists, do not administer the vaccine. Since the first positive results on vaccines have come out, a lot of people have asked me if i think everyone should take them? It may come sooner than we think. Download a blank covid vaccine card in pdf form. It was authorized in the eu on march 11 but the rollout of the shots has not started yet. We inform you that the privacy policy at the remote site can be different from the covid19vaccinejanssen.com website.

Since the first positive results on vaccines have come out, a lot of people have asked me if i think everyone should take them?

Guía técnica 10 de agosto 2021.

If either of these conditions exists, do not administer the vaccine.
Food and drug administration issued an emergency use authorization (eua) for the third vaccine for the prevention.

We inform you that the privacy policy at the remote site can be different from the covid19vaccinejanssen.com website.

Guía técnica 10 de agosto 2021.

The emergency use of this product is only.

Authorized the janssen shot in late february and has administered nearly 5 million doses as of.
Arrangements for the supply of the vaccine are the responsibility of national authorities.

An adverse event is a medical incident that may occur following immunization.

The emergency use of this product is only.

Guía técnica 10 de agosto 2021.

Guía técnica 10 de agosto 2021.

Guía técnica 10 de agosto 2021.

Download a blank covid vaccine card in pdf form.

An adverse event is a medical incident that may occur following immunization.

The janssen covid‑19 vaccine has not been approved or licensed by the u.s.

The cause is not necessarily related to the vaccine.
If either of these conditions exists, do not administer the vaccine.

It was authorized in the eu on march 11 but the rollout of the shots has not started yet.
Acip lifts pause on the administration of janssen/johnson & johnson vaccines.
On february 27, 2021, the u.s.

Download a blank covid vaccine card in pdf form.

Arrangements for the supply of the vaccine are the responsibility of national authorities.

On february 27, 2021, the u.s.

It may come sooner than we think.

Unless approved or licensed by the relevant health authority, the product is investigational and its safety and efficacy have not been established.